Hydrogen in the Built Environment
The expansion of the hydrogen market has opened the door to the possibility of utilizing hydrogen-produced energy in the built environment via fuel cells.

With the pursuit of clean energy continuing to gain traction, hydrogen has been receiving more attention as a possible solution.
Technology for both hydrogen production and its corresponding fuel cells has developed substantially over the past two decades.
Hydrogen Production and Procurement
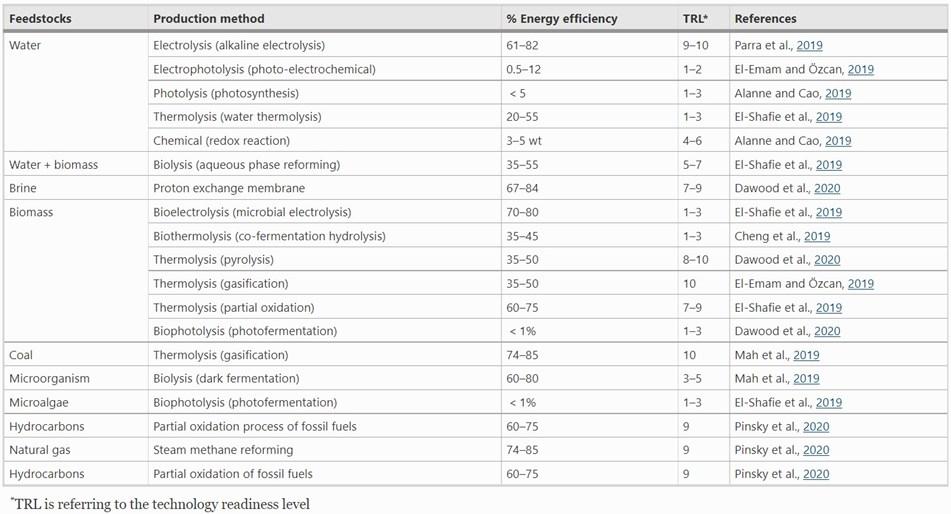
While hydrogen is a common element on earth, it primarily exists in compounds with other elements. To be used in fuel cells, hydrogen needs to be isolated into pure hydrogen molecules (H2). There are several feedstock sources and production methods for hydrogen generation. When looking at feedstocks, hydrogen can be produced from fossil fuels, such as synthesis gas, biomass, water, or ethanol.1 In terms of production methods, there are two main types: thermal processes (reforming) and electrolytic processes (electrolysis/hydrolysis), with several other technologies in development to produce hydrogen from light or biological reactions of microalgae.2
Thermal Processes
The most common method of hydrogen production is steam methane reforming. This method uses high-temperature steam and methane, primarily from natural gas feedstocks, to produce hydrogen.3 The downside of this process is that it also produces carbon monoxide (CO) and carbon dioxide (CO2) as byproducts, contributing to climate change.4
Partial oxidation creates hydrogen via a reaction of methane with oxygen.5 Much like steam methane reforming, partial oxidation creates both CO and CO2 as byproducts. While partial oxidation produces slightly less hydrogen than steam methane reforming, it does not require heat for the reaction to occur and can be done in a smaller reactor vessel.6 These processes can work with other hydrocarbons besides methane, have a high hydrogen yield efficiency (60-85%), and are mature technologies that are already implemented on a large scale.7
Electrolysis
Considered to be the more environmentally conscious method, electrolysis occurs when water is split into hydrogen (H2) and oxygen (O2) gases using electricity.8 Since this process is powered by electricity, it can be carbon neutral if it uses renewable energy sources. Currently, electrolysis has a high efficiency, and is a mature technology that can be implemented on a large scale.9 The major downside of this process is the cost and the low availability of excess renewable electricity.10 The expected growth in the hydrogen market will help to decrease commercial electrolysis and increase its public implementation.

Hydrogen is classified by color to identify how it was produced. There are five main color codes: green for hydrogen made by renewable electricity, brown for coal feedstocks, grey for natural gas or petroleum feedstocks, blue for brown or grey hydrogen paired with carbon capture and storage (CCS), and pink for hydrogen produced by nuclear power.11
Hydrogen’s Production of Energy
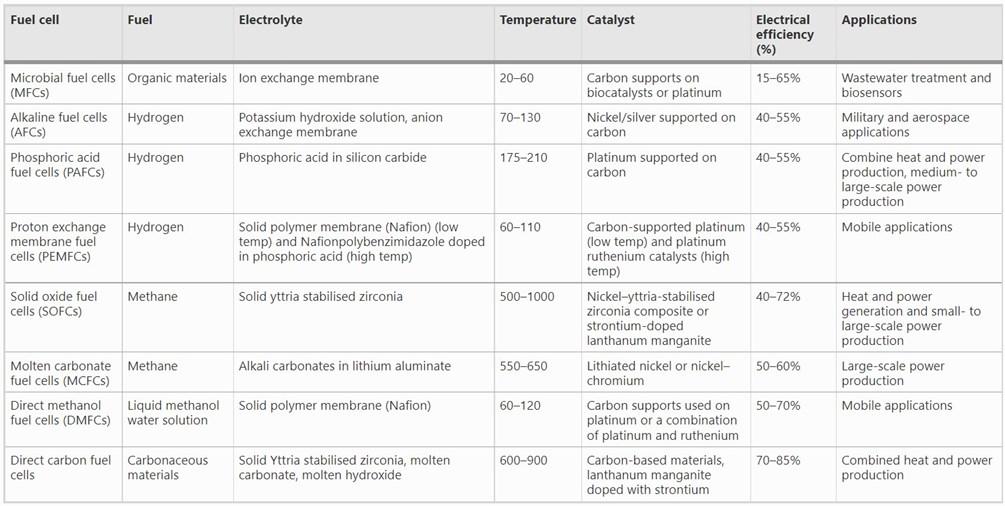
After the hydrogen is produced, it is pumped into the onsite fuel cell or cell stack. Fuel cells break hydrogen into protons and electrons, with the latter creating electricity.12 At the end of the electrical circuit, the electrons reunite with the protons and mix with air to produce end products of water and heat.13 The fuel-to-energy efficiency of hydrogen fuel cells is around 60%, but it could reach up to 90% with heat recovery systems.14 There are several types of fuel cells that differ in operating temperature, efficiency, and maximum power generation.15 Hydrogen fuel cell systems come in various sizes, ranging from a few kW up to several MW, making them viable for a large array of applications.16
Hydrogen’s Application in the Built Environment
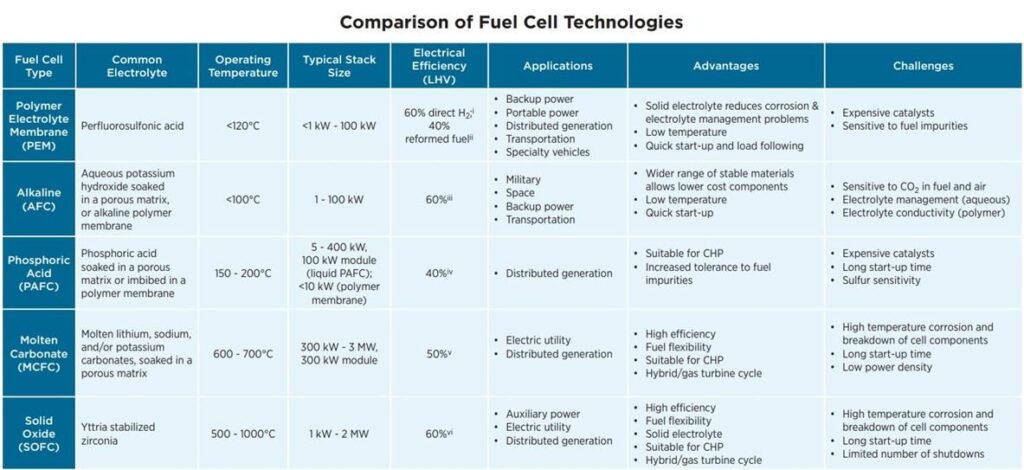
Combining hydrogen fuel cells with heat recovery technologies to form a CHP system is the best option for hydrogen in the built environment. With applications in both energy and heating, hydrogen has a strong potential to be introduced more broadly within the built environment. Already in European countries, hydrogen is beginning to expand within the sector. According to the European Union’s PACE project, over 10,000 fuel cells have been sold within their project’s scope, with projects already underway.17 There are even operational projects in the United States, such as the natural gas fuel cell at the Santa Rita Jail in Dublin, California. The jail paired an onsite fuel cell with its rooftop solar array to decrease its emissions by almost 3,200 tons of GHG each year.18 The fuel cell generates 50% of the jail’s energy needs, 18% of its heating needs using combined heat and power, and has an overall efficiency rating of 58%.19
Environmental Impacts
The production of hydrogen and its use in energy production has been generally regarded as a green alternative for energy production, with some drawbacks. Drawbacks include hydrogen leakages and production, as well as freshwater depletion. European research has found that “a global hydrogen economy with a leakage rate of 1% of the produced hydrogen would produce a climate impact of 0.6% of the fossil fuel system is replaces.”20
However, this may be an optimistic estimation of hydrogen leakage rates and the associated climate impacts, as other research has found that it is expected to increase to 2.9-5.6% by 2050.21 Hydrogen leaks contribute to climate change by reacting with tropospheric OH radials to produce methane and tropospheric ozone.22 Leakage can be a concern, as hydrogen can cause pipes to become brittle, leading to the possibility of leaks in its transport.23 These leakages can lead to gas accumulation and explosions.24 Hydrogen production has also been associated with the following environmental impacts: metal depletion potential, particulate matter formation potential, land use, and terrestrial and freshwater ecotoxicity.25
When looking at the more specific methods of hydrogen production, steam methane reforming and hydrolysis each have their own specific environmental impacts. Utilizing fossil fuels in hydrogen production contributes to climate change via the emission of greenhouse gases. In the case of hydrolysis hydrogen production, this method can be paired with renewable energy sources to be emission-free. However, this method requires a large quantity of freshwater, an already limited resource. Salt water is a more affordable and abundant substitute, but it is riskier due to the corrosion of the metal anode by chloride ions.26
Pros & Cons of Hydrogen and Fuel Cells
Pros
- High efficiency in both production methods and in fuel cells, especially when combined with heat recovery technologies.
- Scalability and size: Fuel cells are much smaller than other renewable power generation sources and can serve a wide variety of needs.
- Emissions: can be carbon-free if generated using renewable-powered electrolysis.
Cons
- Hydrogen is expensive and difficult to transport. It requires incredibly high pressures when transported as a gas or incredibly low temperatures when transported as a liquid.
- Hydrogen embrittlement: due to hydrogen’s small size, it can infiltrate a metal pipe structure and make the metal more susceptible to cracking.
- Hydrogen leaks: leaks are more likely due to hydrogen atoms’ small size. In the atmosphere, it functions as an indirect greenhouse gas (GHG) to produce methane and tropospheric ozone, both GHGs.
- Most hydrogen is currently made from fossil fuels such as natural gas. These processes yield carbon dioxide, a GHG.
- Current costs and availability: Hydrogen is still an expensive energy option, and there is not a large market for it yet, although the market is expected to expand in the coming years.
References
- NREL. “Hydrogen Production and Delivery.” Hydrogen and Fuel Cells, 2016, https://www.nrel.gov/hydrogen/hydrogen-production-delivery.html. Accessed 27 July 2022. ↩︎
- Office of Energy Efficiency and Renewable Energy. “Hydrogen Fuel Basics.” Hydrogen and Fuel Cell Technologies Office, https://www.energy.gov/eere/fuelcells/hydrogen-fuel-basics. Accessed 27 July 2022. ↩︎
- Office of Energy Efficiency and Renewable Energy. “Hydrogen Production: Natural Gas Reforming.” Hydrogen and Fuel Cell Technologies Office, https://www.energy.gov/eere/fuelcells/hydrogen-production-natural-gas-reforming#:~:text=In%20steam%2Dmethane%20reforming%2C%20methane,for%20the%20reaction%20to%20proceed. Accessed 27 July 2022. ↩︎
- Ibid. ↩︎
- Ibid. ↩︎
- Ibid. ↩︎
- Osman, Ahmed I., et al. “Hydrogen production, storage, utilization and environment impacts: a review.” Environmental Chemistry Letters, vol. 20, 2022, pp. 153-188. SpringerLink, https://link.springer.com/article/10.1007/s10311-021-01322-8 ↩︎
- Office of Energy Efficiency and Renewable Energy. “Hydrogen Production Electrolysis.” Hydrogen and Fuel Cell Technologies Office, https://www.energy.gov/eere/fuelcells/hydrogen-production-electrolysis. Accessed 27 July 2022. ↩︎
- Ibid. ↩︎
- Ibid. ↩︎
- EIA. “Hydrogen Explained: Production of Hydrogen.” EIA, https://www.eia.gov/energyexplained/hydrogen/production-of-hydrogen.php#:~:text=To%20produce%20hydrogen%2C%20it%20must,electrolysis%20(splitting%20water%20with%20electricity. Accessed 27 July 2022. ↩︎
- Office of Energy Efficiency and Renewable Energy. “Fuel Cells.” Hydrogen and Fuel Cell Technologies Office, https://www.energy.gov/eere/fuelcells/fuel-cells. Accessed 20 September 2022. ↩︎
- Ibid. ↩︎
- Fuel Cell and Hydrogen Energy Association. “Stationary Power.” Fuel Cell and Hydrogen Energy Association, https://www.fchea.org/stationary. Accessed 27 July 2022. ↩︎
- U.S. Department of Energy Federal Energy Management Program. “Fuel Cells and Renewable Hydrogen.” Whole Building Design Guide, 21 October 2016, https://www.wbdg.org/resources/fuel-cells-and-renewable-hydrogen. ↩︎
- Ibid. ↩︎
- PACE. “Fuel cells and hydrogen in buildings are ready to address the challenges of our energy system.” PACE, 24 June 2019, https://pace-energy.eu/fuel-cells-and-hydrogen-in-buildings-are-ready-to-address-the-challenges-of-our-energy-system/. ↩︎
- County of Alameda, California. “Santa Rita Jail Fuel Cell Power Plant.” County of Alameda, CA, 2006, https://www.acgov.org/sustain/documents/fuelcellfactsheet.pdf. ↩︎
- Ibid. ↩︎
- Derwent, Richard, et al. “Global Environmental Impacts of the Hydrogen Economy.” International Journal of Nuclear Hydrogen Production and Applications. 20 May 2006. https://www.geos.ed.ac.uk/~dstevens/Presentations/Papers/derwent_ijhr06.pdf. ↩︎
- Fan, Zhiyuan, et al. “Hydrogen Leakage: A Potential Risk for the Hydrogen Economy.” Columbia Center on Global Energy Policy. 5 July 2022. https://www.energypolicy.columbia.edu/research/commentary/hydrogen-leakage-potential-risk-hydrogen-economy. ↩︎
- Derwent et al. ↩︎
- Osman et al. ↩︎
- Ibid. ↩︎
- Ibid. ↩︎
- Ibid. ↩︎
